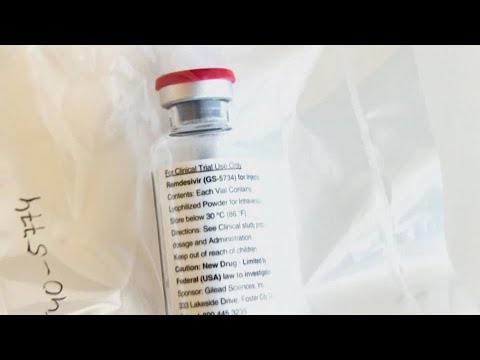
吉利德的雷米替西韋獲得美國FDA準許 (Gilead's remdesivir gets U.S. FDA approval)
 沒有此條件下的單字
沒有此條件下的單字US /əˈprəʊtʃ/
・
UK /ə'prəʊtʃ/
- v.t./i.逼近;找...商量
- n. (c./u.)通道;入口;接洽;處理方式;方法
US /pænˈdɛmɪk/
・
UK /pæn'demɪk/
- adj.(疾病)大規模流行的,廣泛蔓延的
- n.大流行病
US / dɪˈbet/
・
UK /dɪ'beɪt/
- n. (c./u.)辯論;爭論;辯論會
- v.t./i.思考;盤算;辯論
US /ɪmˈpruv/
・
UK /ɪm'pru:v/