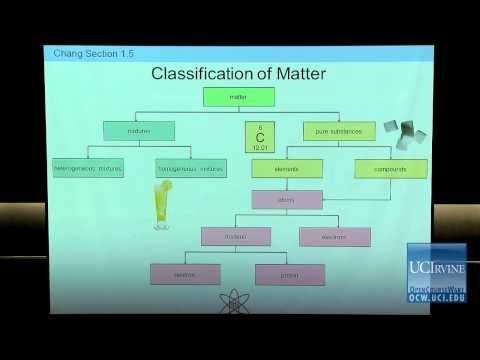
普通化學1P的準備。第02講。物質的分類。 (Preparation for General Chemistry 1P. Lecture 02. Classification of Matter.)
Amy.Lin 發佈於 2021 年 01 月 14 日  沒有此條件下的單字
沒有此條件下的單字US /ˌɪndəˈvɪdʒuəl/
・
UK /ˌɪndɪˈvɪdʒuəl/
- n. (c.)個人;單個項目;個體;個人賽
- adj.個人的;獨特的;個別的;獨特的
US /ɪk'strimlɪ/
・
UK /ɪkˈstri:mli/
- adv.極端地 ; 非常地;非常;從極端的角度來看
US /məˈtɪriəl/
・
UK /məˈtɪəriəl/
- n. (c./u.)布料;素材;資料;材料;物質
- adj.重要的;物質的
US /ˈmʌltəpəl/
・
UK /ˈmʌltɪpl/
- adj.多重的;多種的;多發性的;多重的
- n. (c.)多;多個的;乘數
- pron.多重的