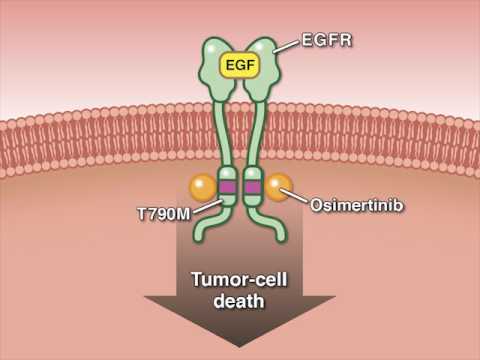
奧希美替尼在晚期非小細胞肺癌中的應用 (Osimertinib in Advanced Non–Small-Cell Lung Cancer)
 沒有此條件下的單字
沒有此條件下的單字US /sɪɡˈnɪfɪkənt/
・
UK /sɪgˈnɪfɪkənt/
US /ˈpɑzɪtɪv/
・
UK /ˈpɒzətɪv/
- adj.積極的;建設性的;確定的;正極的;積極的;有利的;陽性的;樂觀的;正數的;正像的
- n.正片
- v.t.宴請;款待;招待;治療;處理;對待;處理
- n. (c./u.)對待 ;;點心, 零嘴;特別的享受
- n. (c./u.)公有地;公共用地;廣場
- adj.共用的;常見的;普通的;普遍的;粗俗的;普通名詞