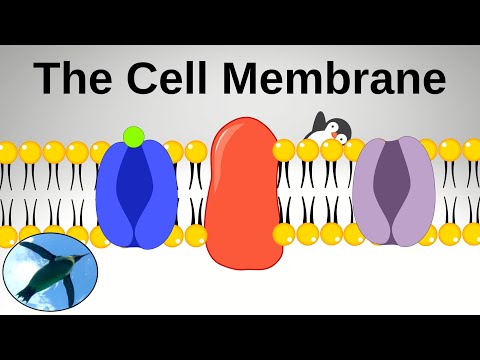
迎接...細胞膜! (Meet... The Cell Membrane!)
Amy.Lin 發佈於 2021 年 01 月 14 日  沒有此條件下的單字
沒有此條件下的單字US /ˈstrʌk.tʃɚ/
・
UK /ˈstrʌk.tʃə/
- n. (c./u.)結構;建築物
- v.t.構成;組織
US /ˈbæriɚ/
・
UK /'bærɪə(r)/
- n.屏障;屏障;障礙物;柵欄;隔閡;障礙;屏障 (電腦)
US /ˈmɑlɪˌkjul/
・
UK /ˈmɒlɪkju:l/
- n. (c./u.)外形;形狀;狀況;狀態;形狀;樣子
- v.t.使成形;塑造;塑造