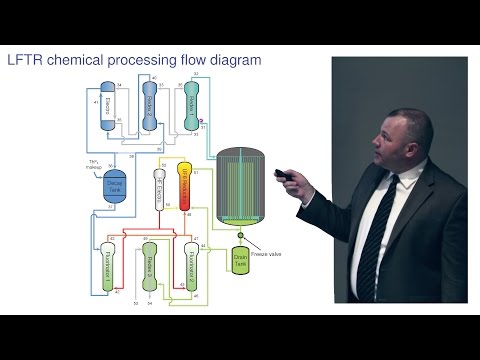
LFTR 化學處理和動力轉換 - Kirk Sorensen。 (LFTR Chemical Processing & Power Conversion - Kirk Sorensen)
Steven Hsu 發佈於 2021 年 01 月 14 日  沒有此條件下的單字
沒有此條件下的單字- n. (u.)節拍;(準確的)時間;時間(多寡);(經歷的)一段時光;(經歷的)時光;時代;時期;時間;時刻;時候
- v.t.測量(節拍);為...計算時機;計時;測量時間;使適時;安排...的時間
- n. (c./u.)人;人們;人們;家人;員工
- v.t.居住
- n. pl.人們
- n. (c./u.)成品;工作的成果;產品;作品;工作;職業;工作(場所);(工作等的)成果
- v.t./i.起作用;行得通;運轉;運作;運行;活動;起作用;有效用;(機器等)運轉;活動
- adj.工作相關的
US /ædˈvæntɪdʒ/
・
UK /əd'vɑ:ntɪdʒ/
- n. (c./u.)優勢;優點;利益
- v.t.利用;佔便宜