字幕與單字
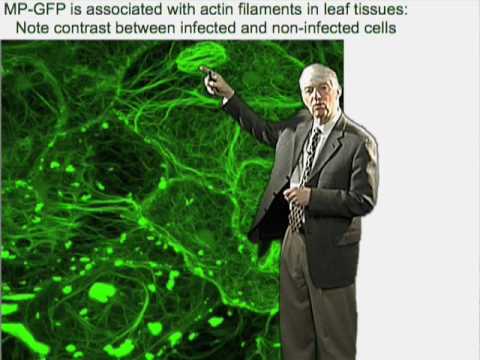
羅傑-比奇(丹佛斯中心)第1部分:植物病毒感染的生物學問題。 (Roger Beachy (Danforth Center) Part 1: Biology of Plant Virus Infection)
00
江子揚 發佈於 2021 年 01 月 14 日收藏
影片單字
movement
US /ˈmuvmənt/
・
UK /ˈmu:vmənt/
- n. (c./u.)樂章;(有特定目標的)運動;社會運動;動作;樂章;運動;變化;發展;機械裝置;排便;(芭蕾)舞步;軍事調動
A2 初級初級英檢
更多 使用能量
解鎖所有單字
解鎖發音、解釋及篩選功能
