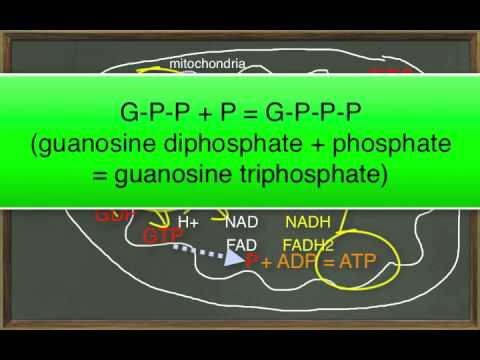
第25章 模塊二 糖酵解、TCA循環和ETS (Chapter 25 Module 2 Glycolysis, TCA Cycle, and ETS)
Cheng-Hong Liu 發佈於 2021 年 01 月 14 日  沒有此條件下的單字
沒有此條件下的單字US /ˈprɑsˌɛs, ˈproˌsɛs/
・
UK /prə'ses/
- v.t.用電腦處理(資料);(依照規定程序)處理;處理;流程;加工;理解
- n. (c./u.)(規定的)程序;過程;進程;方法;法律程序;進程
US /ˈmɑlɪˌkjul/
・
UK /ˈmɒlɪkju:l/
- n.呼喚;召喚;叫聲;判定;判決;拜訪;短時間逗留;(裁判的)判決
- v.t./i.罷工;拜訪某人;拜訪某地;打電話;叫喊;大叫;大喊
- v.i.叫喊;呼叫
- v.t.舉行選舉;召集會議;判決;稱為;叫作;判定
US /ˈsɪstəm/
・
UK /'sɪstəm/
- n. (c./u.)系統;體系;方法;(身體)系統;(電腦)系統
- adj.系統