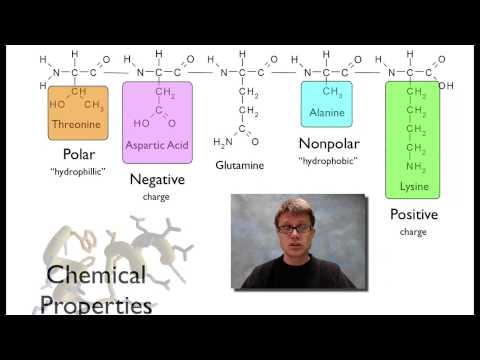
蛋白質 (Proteins)
Cheng-Hong Liu 發佈於 2021 年 01 月 14 日  沒有此條件下的單字
沒有此條件下的單字US /ˈbesɪkəli,-kli/
・
UK /ˈbeɪsɪkli/
US /ˈnɛɡətɪv/
・
UK /'neɡətɪv/
- n.負電極的;否定詞;否定句;底片
- adj.消極的;負的;負面的;否定的;陰性的;負電的
US /ˈpɑzɪtɪv/
・
UK /ˈpɒzətɪv/
- adj.積極的;建設性的;確定的;正極的;積極的;有利的;陽性的;樂觀的;正數的;正像的
- n.正片
US /ˈstrʌk.tʃɚ/
・
UK /ˈstrʌk.tʃə/
- n. (c./u.)結構;建築物
- v.t.構成;組織