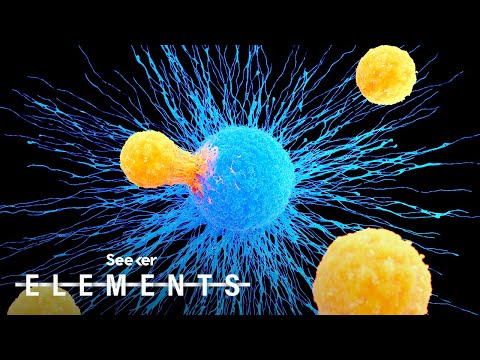
This Cell Might Hold the Answer to a Universal Cure for Cancer
Summer 發佈於 2020 年 08 月 28 日  沒有此條件下的單字
沒有此條件下的單字US /spɪˈsɪfɪk/
・
UK /spəˈsɪfɪk/
US /pəˈtɛnʃəl/
・
UK /pəˈtenʃl/
- adj.可能的;潛在的;潛在的
- n. (u.)潛力,潛能
- n. (c./u.)潛力;潛能;潛在候選人;勢
US /ɪˈmjoon/
・
UK /ɪˈmju:n/
US /ˈrek.əɡ.naɪz/
・
UK /ˈrek.əɡ.naɪz/
- v.t.認可;接受;賞識;承認;表彰;嘉獎;認出,認識