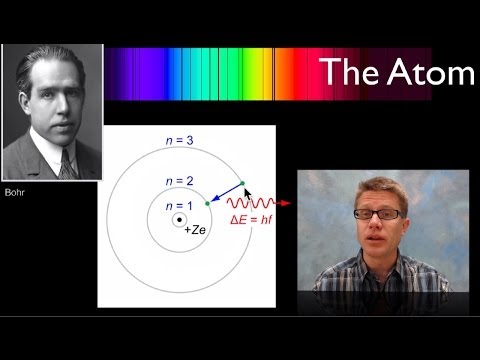
玻爾原子 (The Bohr Atom)
Bravo001 發佈於 2021 年 01 月 14 日  沒有此條件下的單字
沒有此條件下的單字US /ˈsɪriz/
・
UK /ˈsɪəri:z/
- n. (c./u.)系列;系列;電視劇;系列賽;級數;系列
- n. pl.一連串;一系列
- adj.串聯
US /ˈspɛktrəm/
・
UK /'spektrəm/
- n.光譜 ; 波譜;(看法、感覺等的)範圍,各層次
- v.t.點燃;用燈光指引
- adj.亮的;亮;輕的;輕鬆的
- n. (c./u.)光線;理解;光;燈;交通號誌;神色
- adv.輕裝地
US /ˈnʌmbɚ/
・
UK /ˈnʌmbə(r)/
- n. (c./u.)數字;短曲;短歌;總數
- v.t.編了號的;給...編號;計入