字幕與單字
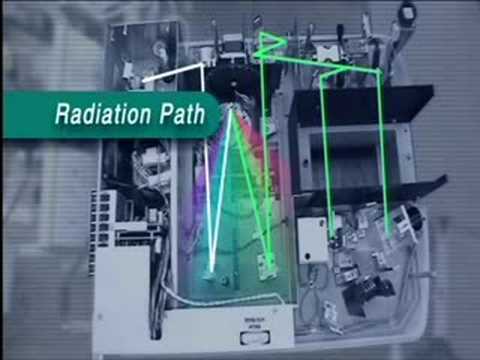
紫外/可見光光譜學(UV-Vis) (Ultraviolet/Visible Spectroscopy (UV-Vis))
00
Cheng-Hong Liu 發佈於 2021 年 01 月 14 日收藏
影片單字
reference
US /ˈrɛfərəns, ˈrɛfrəns/
・
UK /'refrəns/
- n.推薦函;參考文獻;參考文獻;參考;提及;參考資料;查閱;引用 (電腦科學);證明人;提交仲裁;參考點;參考書
- v.t.參考;參考
- prep.關於
A2 初級多益中級英檢
更多 使用能量
解鎖所有單字
解鎖發音、解釋及篩選功能
