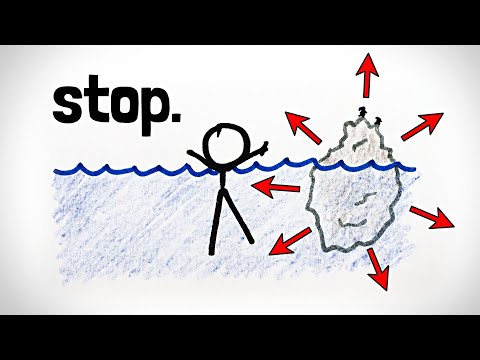
凍結的水會膨脹。如果你不讓它膨脹呢? (Freezing water expands. What if you don't let it?)
Summer 發佈於 2022 年 10 月 14 日  沒有此條件下的單字
沒有此條件下的單字- adv.按照字面上地;(用于强调)确实地,真正地;幾乎
US /ɛnˈtaɪr/
・
UK /ɪn'taɪə(r)/
- adj.全體的 ; 完全的;未分割的;全緣的 (植物學)
US /ɪˈvɛntʃuəli/
・
UK /ɪˈventʃuəli/
US /ˈkɑnˌtɛnt/
・
UK /'kɒntent/
- adj.滿足的;滿意的
- n. (c./u.)內容;主題;內容;滿意;內容 (數位);含量
- v.t.使…滿足
- v.i.同意