字幕與單字
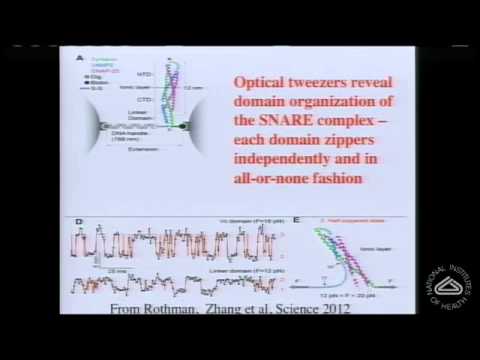
突觸處神經遞質同步釋放的分子機制 (The Molecular Mechanism of Synchronous Neurotransmitter Release at Synapses)
00
Precious Annie Liao 發佈於 2021 年 01 月 14 日收藏
影片單字
release
US /rɪ'li:s/
・
UK /rɪ'li:s/
- v.t.釋放;解放;(電影音樂等)上市;釋放;放手;發布
- n.釋放;解放(的動作);發行(新產品、電影、書籍);解放(從悲傷或困境中);豁免;釋放;釋放裝置;棄權書;新聞稿;版本
A2 初級多益中級英檢
更多 使用能量
解鎖所有單字
解鎖發音、解釋及篩選功能
